By Steve Pak, | February 27, 2016
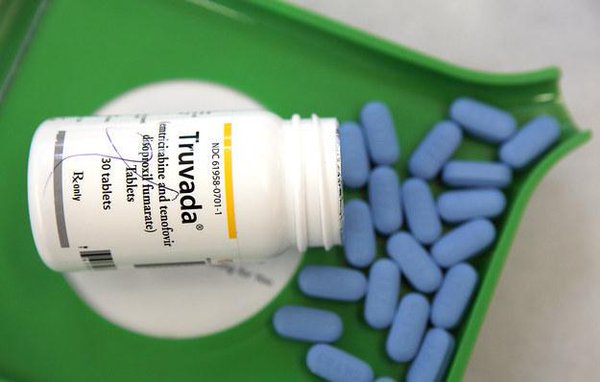
Truvada Anti-HIV Drug
Truvada reportedly failed as a pre-exposure prophylaxis (PrEP) to prevent human immunodeficiency virus (HIV) in a daily user of the anti-HIV drug. Researchers have reported the first documented case in which a regular PrEP user contracted a drug-resistant strain of the disease that can develop into acquired immune deficiency syndrome (AIDS).
Like Us on Facebook
Lead author Dr. David Knox is an HIV specialist at Maple Leaf Medical Clinic. He presented the case study's findings at this year's Conference on Retroviruses and Opportunistic Infections (CROI) in Boston.
The scientists in the study concluded that PrEP users can still get HIV when a strain is resistant to the two drugs in Truvada. Those are tenofovir and emtricitabine. However, PrEP experts believe that cases in which the anti-HIV drugs fail will still be rare.
Researchers' case study was based on a Canadian gay man. The 43-year-old had long-term PrEP use, yet after two years on Truvada he tested positive for HIV and the first tests showed that he had recently been infected, according to POZ.
The man had tested positive for the p24 antigen (substance that helps produce antibodies). It shows up in tests about three weeks after an HIV infection and then disappears a couple of weeks afterwards.
At the time the Canadian man tested positive for p24 he tested negative for HIV antibodies, which usually appear 2-8 weeks after a viral infection.
Experts estimate that gay men who take Truvada four or more times per week have a 99 percent change of not getting HIV. The Centers for Disease Control and Prevention (CDC) recommend taking Truvada daily for the best results, but the drug seems to be effective when users sometimes miss a dose.
Scientific studies have shown Truvada is very effective in HIV prevention. None of the over 1,400 high-risk men in a Kaiser Permanent program have contracted HIV yet. They even have a high rate of other sexually transmitted diseases (STDs) including hepatitis C.
In other HIV/AIDS drug news last year Turing Pharmaceuticals was criticized for increasing an AIDS drug from $13 to $750 per pill. It had bought the rights for the drug Daraprim for $55 million, according to Daily Mail.
Here's an explanation of PrEP:
-
Use of Coronavirus Pandemic Drones Raises Privacy Concerns: Drones Spread Fear, Local Officials Say

-
Coronavirus Hampers The Delivery Of Lockheed Martin F-35 Stealth Fighters For 2020

-
Instagram Speeds Up Plans to Add Account Memorialization Feature Due to COVID-19 Deaths

-
NASA: Perseverance Plans to Bring 'Mars Rock' to Earth in 2031

-
600 Dead And 3,000 In The Hospital as Iranians Believed Drinking High-Concentrations of Alcohol Can Cure The Coronavirus

-
600 Dead And 3,000 In The Hospital as Iranians Believed Drinking High-Concentrations of Alcohol Can Cure The Coronavirus

-
COVID-19: Doctors, Nurses Use Virtual Reality to Learn New Skills in Treating Coronavirus Patients